
In August 2022, the Department of Health and Human Services (HHS) declared mpox a public health emergency. To a public already exhausted by the COVID-19 pandemic, the news that another disease now also constituted an emergency was frightening.
Related to smallpox, mpox most commonly presents as skin lesions and flu-like symptoms with an accompanying, painful rash of pus-filled blisters. The sores and oral fluids of an infected person can transmit the virus easily through close physical contact. At the time of the emergency declaration, mpox could only be diagnosed with time-consuming laboratory-based tests, making doctors and anxious patients wait days for results. Widespread access to testing in point-of-care settings like doctors’ offices and, even more conveniently, at home would be an important weapon to stop the spread of this new epidemic. The bad news was that no such tests existed… yet.
The good news? The Rapid Acceleration of Diagnostics Technology (RADx® Tech) Independent Technology Assessment Program (ITAP), established by the National Institutes of Health (NIH) and the U.S. Food and Drug Administration (FDA) during the COVID-19 pandemic, offered an infrastructure to accelerate the regulatory review process and potential commercialization of rapid, accurate tests at the necessary scale. Designed with a funnel structure, ITAP solicits ideas for new technology from diagnostics and medtech innovators, selects viable proposals, and provides validation, de-risking, and scale-up support to rapidly bring multiple solutions to market.
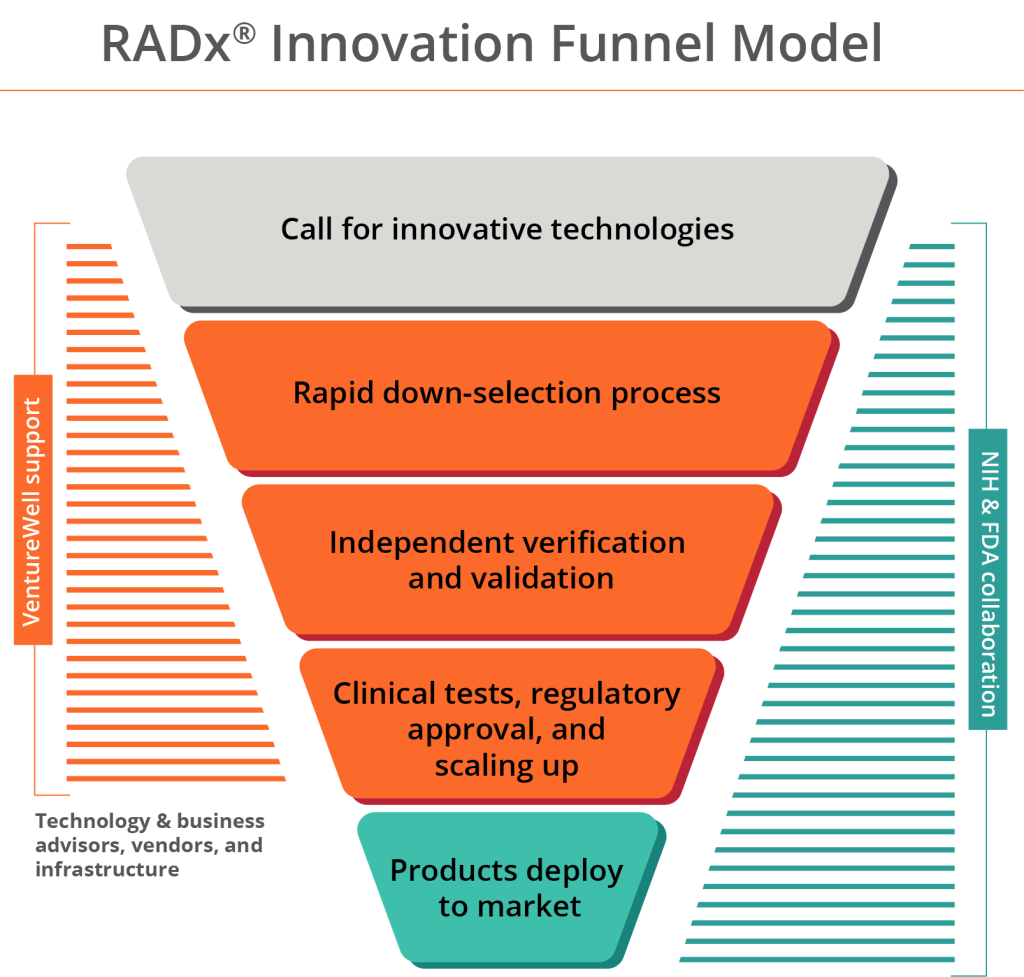
VentureWell, with decades of innovation management expertise, is a key partner in this effort, from the top of the funnel to the bottom. VentureWell allows medical device companies and their in-house teams to move technologies more quickly to consumers by providing access to technical, business, regulatory, and other commercialization experts, as well as a roster of vendors who provide commercialization services.
“We provide behind-the-scenes support that makes sure everyone has what they need,” explains Rebekah Neal, Ph.D., vice president of Commercialization at VentureWell, who oversees its participation in ITAP. While scientists made medical breakthroughs and government agencies provided funding and support, VentureWell was the connective tissue that held the project together and kept it moving. “There are so many points in the process where problems with organization or workflow can grind everything to a halt. VentureWell steps in and smooths the way, moving quickly and effectively to remove barriers and manage the details.”
Solving the Problem of At-Home Testing with RADx® ITAP
In April 2020, as the COVID-19 virus spread across the U.S., the NIH launched the RADx® Tech program within the National Institute of Biomedical Imaging and Bioengineering (NIBIB). The objective of the RADx® Tech program was to develop a robust research & development pipeline of innovative COVID-19 diagnostic technologies to increase national testing capacity and optimize technology performance across the testing landscape.
ITAP—launched in the fall of 2021—was an extension of RADx®, bringing together the FDA and experts from HHS in a coordinated, collaborative push to quickly develop and validate solutions, first for COVID-19 diagnostics, and then for a range of additional diagnostic challenges. Innovators who progressed through the validation phase submitted their solution for regulatory review and, if successful, achieved emergency use authorization.
“RADx showed us the power of collaboration. When we bring together the best of government, academia, and the private sector, we can solve intractable problems with breathtaking speed.”
— Rebekah Neal, Ph.D., vice president of Commercialization at VentureWell
Just one month after the HHS emergency declaration, the ITAP for mpox project kicked off. Three NIBIB partners provided ITAP with foundational support and back-end management that smoothed over the challenges of collaboration and expedited commercialization. VentureWell sourced and managed the expert consultants, program managers, and other essential individuals who implemented the program. Cimit, a nonprofit dedicated to tackling difficult health challenges, provided program and process coordination. The Validation Center at Emory University and its partner organizations, Children’s Healthcare of Atlanta and Georgia Institute of Technology, provided verification and validation support, including rapid access to analytical, clinical, engineering, structural biology, and human factors evaluations of new diagnostics.
Two months in, ITAP scientists had acquired the clinical samples they needed for independent testing. The window from the emergency declaration in August 2022 to FDA emergency use authorization of an mpox test was a mere six months—prior to ITAP, authorization or clearance of new tests could easily take years.
The Power of Interagency Collaboration
The mpox test was not the first success credited to ITAP’s leadership in interagency collaboration—its first goal was to produce hundreds of millions of COVID-19 tests per month. ITAP—supported by VentureWell, Cimit, and Emory University—moved at record speed, condensing a process that usually takes years into months. Today, at-home COVID-19 tests are readily available for those who need them.
Read more about how RADx® boosted the ability of the U.S. to produce COVID-19 tests.
“RADx® showed us the power of collaboration. When we bring together the best of government, academia, and the private sector, we can solve intractable problems with breathtaking speed,” says Neal.
Testing is now much more accessible for those who believe they have been exposed to mpox, because of the framework established by the push to provide at-home COVID-19 tests. The first point-of-care test for mpox offers results in under an hour. In a single visit, healthcare providers can give patients an accurate diagnosis based on the test and advise on next steps. This minimizes community transmission and maximizes treatment options. For those with the disease, it can result in less suffering and fewer complications. The second authorization, of an ITAP-supported home collection kit, offers the privacy and convenience of sample collection without visiting a doctor.
Preparing for the Next Pandemic
The COVID-19 pandemic taught us the importance of establishing a systematic approach to pandemic preparedness. The speed of healthcare innovation has increased, as has the pace at which we can bring new solutions—vaccines, treatments, tests—from the laboratory to the doctor’s office, and even into schools, businesses, and homes.
VentureWell plays a key role in providing the backbone of support needed for efficient coordination and collaboration. The COVID-19 pandemic pushed us to evolve our response to a major public health emergency, and then the mpox epidemic tested whether the process could be replicated. The ITAP model that has been developed means we’re better prepared now to respond to future challenges, by working together, whether for COVID-19, mpox, or future diseases.
VentureWell serves as the NIH/NIBIB (National Institute of Biomedical Imaging and Bioengineering) Innovation Funnel—Commercialization Center. Learn more about how we’re accelerating the development of innovative healthtech solutions.
The Independent Test Assessment Program (ITAP) for mpox was funded in part with Federal funds from the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health, Department of Health and Human Services, under Contract Nos. 75N92022D00013, 75N92022D00015, and 75N92022D00014.