Massachusetts Institute of Technology
2014 BMEIdea Second Prize Winner, winning $5,000
Stage 1 & 2 E-Team grantee, $25,000
A new drug delivery technology that will simplify drug reconstitution between a lyophilized (freeze-dried) drug and water at the point of care. It is designed for patients who regularly inject lyophilized drugs such as hCG, insulin or HGH.
http://www.recontherapeutics.com
The team members:
Christopher Lee, 24, Ann Arbor, MI;
John Lewandowski, 24, Shaker Heights, OH;
Ben Maimon, 24, Westfield,. NJ;
Babak Movassaghi, 39, Dusseldorf, Germany;
Ho-Jun Suk, 28, Incheon, South Korea
The device:
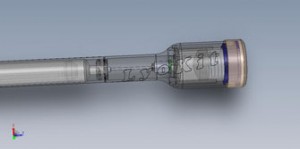
LyoKit is a drug delivery technology designed for patients who regularly inject lyophilized drugs such as hCG, insulin or HGH. This technology simplifies drug reconstitution between a lyophilized (freeze-dried) drug and sterile solvent. The LyoKit device couples a standard off-the-shelf syringe with an external pressurized water chamber, resulting in a simple one-step reconstitution process. The device is customizable to a full range of biologics and can be rapidly adopted to market with minimal regulatory concerns.
The problem:
The gold standard for at-home, self-injection of large biologics involves providing a patient with a vial of drug and a vial of injection solvent. The process of preparing a dose involves 5-10 steps, and necessitates the use of 2-4 syringes and needles, transfer vials, and patient calculations leading to frequent mistakes and decreased compliance. In addition, unused doses require immediate refrigeration, which is extremely inconvenient.
The solution:
Recon Therapeutics has developed a new drug delivery technology that simplifies drug reconstitution between a lyophilized drug and sterile solvent at the point of care. The Lyokit Drug Reconstitution System differentiates itself by being highly intuitive to use, reconstituting biologics with simple one finger actuation. In addition, the reconstitution process occurs using an external pressurized chamber, enabling reconstitution of biologics with a wide spectrum of solubility profiles. Lastly, the Lyokit is an all-inclusive kit that encompasses injection site sterility, needle safety, and disposal all in one embodiment. Coupled with our business model of integrating the system with off-the-shelf syringes, Recon can provide a cost advantage of up to 60% to both the customer and pharmacy, enabling Recon Therapeutics to access an annual market for drug reconstitution technologies exceeding $1.8 billion.
Challenges encountered:
According to team member Christopher Lee, “As a team we have faced many challenges in distilling our regulatory pathway and getting in touch with the correct experts in this domain. In addition, we also initially struggled to determine what type of products that require reconstitution should be targeted with our device first.”
Accomplishments and progress to date:
Recon Therapeutics has incorporated as a LLC and filed a full utility patent application. They have completed a first pass design freeze and developed a beta prototype, which has been designed for manufacturing. Critically, Recon has secured additional funding as an MIT Global IDEAS winner and Harvard Innovation Labs Dean’s Health Life Science Challenge Finalist. They have also established additional working relationships with sterilization facilities, compounding pharmacies, and injection molders. Through Men’s Health Boston, the team spoke with patients and nurses, and was able to perform several rounds of usability and human factors studies.
Their tip for other student innovators:
Always try to do something more with a student project, don’t let it just sit on a shelf.